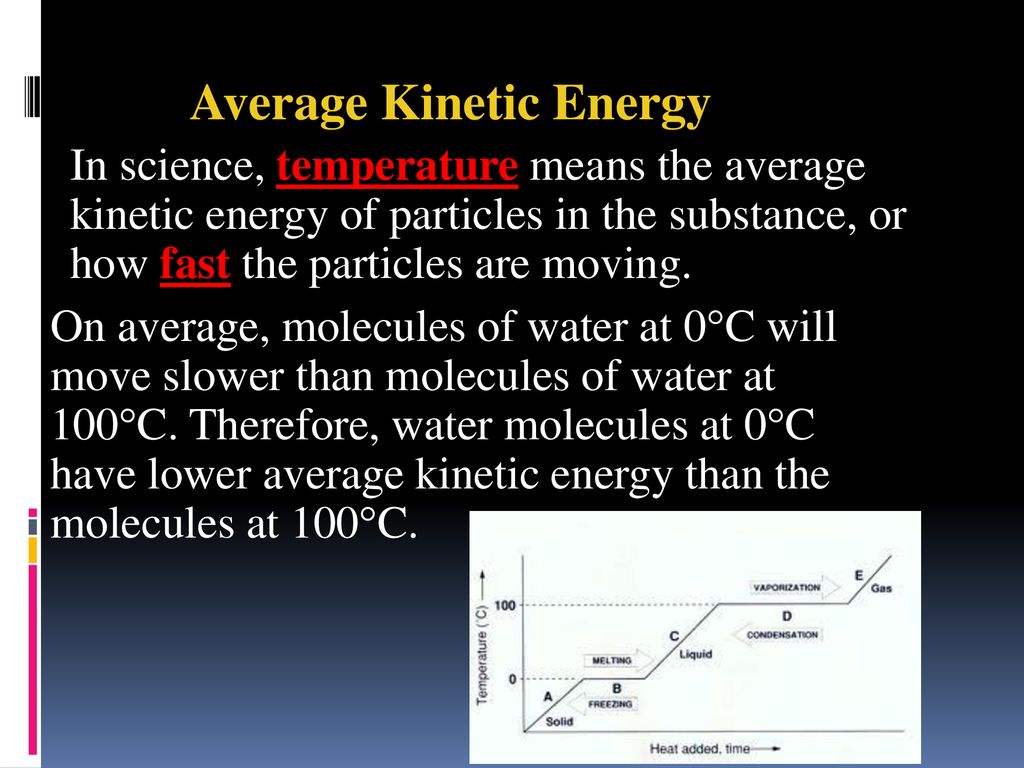
How Does Volume Affect Average Kinetic Energy . the average kinetic energy of the molecules of any gas depends on only the temperature, and at a given temperature, all gaseous. The test of the kmt. the kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. At room temperature, the molecules in a sample of liquid water have the same. at a given temperature, the particles of any substance have the same average kinetic energy.
from slideplayer.com
at a given temperature, the particles of any substance have the same average kinetic energy. The test of the kmt. the average kinetic energy of the molecules of any gas depends on only the temperature, and at a given temperature, all gaseous. At room temperature, the molecules in a sample of liquid water have the same. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. the kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance.
Solids, Liquids, and Gases ppt download
How Does Volume Affect Average Kinetic Energy the kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. at a given temperature, the particles of any substance have the same average kinetic energy. the average kinetic energy of the molecules of any gas depends on only the temperature, and at a given temperature, all gaseous. The test of the kmt. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. the kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. At room temperature, the molecules in a sample of liquid water have the same.
From www.youtube.com
Calculate Average Energy, Total Transitional Energy How Does Volume Affect Average Kinetic Energy at a given temperature, the particles of any substance have the same average kinetic energy. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. At room temperature, the molecules in a sample of liquid water have the same. the kelvin temperature of a substance is directly proportional to the. How Does Volume Affect Average Kinetic Energy.
From www.slideserve.com
PPT GASES PowerPoint Presentation, free download ID4200087 How Does Volume Affect Average Kinetic Energy The test of the kmt. At room temperature, the molecules in a sample of liquid water have the same. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. the kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. at. How Does Volume Affect Average Kinetic Energy.
From www.youtube.com
Derivation of relationship between energy per unit volume and How Does Volume Affect Average Kinetic Energy the kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. the average kinetic energy of the molecules of any gas depends on only the temperature, and at a given temperature, all gaseous. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of. How Does Volume Affect Average Kinetic Energy.
From www.slideserve.com
PPT Chapter 11 Molecular Composition of Gases PowerPoint How Does Volume Affect Average Kinetic Energy At room temperature, the molecules in a sample of liquid water have the same. The test of the kmt. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. the average kinetic energy of the molecules of any gas depends on only the temperature, and at a given temperature, all gaseous.. How Does Volume Affect Average Kinetic Energy.
From slideplayer.com
Solids, Liquids, and Gases ppt download How Does Volume Affect Average Kinetic Energy the kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. the average kinetic energy of the molecules of any gas depends on only the temperature, and at a given temperature, all gaseous. At room temperature, the molecules in a sample of liquid water have the same. The test. How Does Volume Affect Average Kinetic Energy.
From www.slideserve.com
PPT ENERGY IGCSE PHYSICS PowerPoint Presentation ID6037934 How Does Volume Affect Average Kinetic Energy the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. the average kinetic energy of the molecules of any gas depends on only the temperature, and at a given temperature, all gaseous. At room temperature, the molecules in a sample of liquid water have the same. at a given temperature,. How Does Volume Affect Average Kinetic Energy.
From www.slideserve.com
PPT Unit 6 PowerPoint Presentation, free download ID1516058 How Does Volume Affect Average Kinetic Energy At room temperature, the molecules in a sample of liquid water have the same. the kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. at a given temperature, the particles of any substance have the same average kinetic energy. the average kinetic energy of the molecules of. How Does Volume Affect Average Kinetic Energy.
From www.slideserve.com
PPT Average Energy of a Molecule PowerPoint Presentation How Does Volume Affect Average Kinetic Energy The test of the kmt. At room temperature, the molecules in a sample of liquid water have the same. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. the kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. at. How Does Volume Affect Average Kinetic Energy.
From haipernews.com
How To Calculate Average Energy Chemistry Haiper How Does Volume Affect Average Kinetic Energy at a given temperature, the particles of any substance have the same average kinetic energy. The test of the kmt. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. the average kinetic energy of the molecules of any gas depends on only the temperature, and at a given temperature,. How Does Volume Affect Average Kinetic Energy.
From www.teachoo.com
Energy Definition, Formula, Examples Teachoo How Does Volume Affect Average Kinetic Energy the kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. the average kinetic energy of the molecules of any gas depends on only the temperature, and at a given temperature,. How Does Volume Affect Average Kinetic Energy.
From schematicrebuchav1.z22.web.core.windows.net
Diagram Of Energy How Does Volume Affect Average Kinetic Energy the average kinetic energy of the molecules of any gas depends on only the temperature, and at a given temperature, all gaseous. at a given temperature, the particles of any substance have the same average kinetic energy. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. At room temperature,. How Does Volume Affect Average Kinetic Energy.
From www.slideserve.com
PPT Chapter 10 States of Matter PowerPoint Presentation, free How Does Volume Affect Average Kinetic Energy the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. at a given temperature, the particles of any substance have the same average kinetic energy. At room temperature, the molecules in a sample of liquid water have the same. the average kinetic energy of the molecules of any gas depends. How Does Volume Affect Average Kinetic Energy.
From engineerfix.com
What Is Energy? Definition, Examples, Equation, and FAQs How Does Volume Affect Average Kinetic Energy At room temperature, the molecules in a sample of liquid water have the same. the kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. The test of the kmt. the average kinetic energy of the molecules of any gas depends on only the temperature, and at a given. How Does Volume Affect Average Kinetic Energy.
From www.slideserve.com
PPT Theory PowerPoint Presentation, free download ID5326508 How Does Volume Affect Average Kinetic Energy the kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. the average kinetic energy of the molecules of any gas depends on only the temperature, and at a given temperature, all gaseous. at a given temperature, the particles of any substance have the same average kinetic energy.. How Does Volume Affect Average Kinetic Energy.
From www.slideserve.com
PPT Chapter 5 Gases PowerPoint Presentation, free download ID6593753 How Does Volume Affect Average Kinetic Energy the average kinetic energy of the molecules of any gas depends on only the temperature, and at a given temperature, all gaseous. The test of the kmt. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. the kelvin temperature of a substance is directly proportional to the average kinetic. How Does Volume Affect Average Kinetic Energy.
From www.slideserve.com
PPT Temperature v. Heat PowerPoint Presentation ID1701293 How Does Volume Affect Average Kinetic Energy the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. The test of the kmt. At room temperature, the molecules in a sample of liquid water have the same. the kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. the. How Does Volume Affect Average Kinetic Energy.
From www.toppr.com
The relation between the gas pressure P and average energy per How Does Volume Affect Average Kinetic Energy the average kinetic energy of the molecules of any gas depends on only the temperature, and at a given temperature, all gaseous. At room temperature, the molecules in a sample of liquid water have the same. the kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. The test. How Does Volume Affect Average Kinetic Energy.
From online-learning-college.com
Gravitational potential and energy Calculating energies How Does Volume Affect Average Kinetic Energy at a given temperature, the particles of any substance have the same average kinetic energy. At room temperature, the molecules in a sample of liquid water have the same. the kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. the average kinetic energy of the gas molecules. How Does Volume Affect Average Kinetic Energy.